 |
|
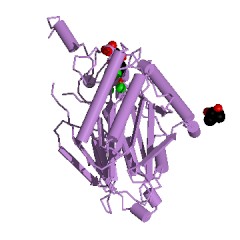
Main view: F 1,6 BPase |
Blood glucose levels are maintained by balancing the amount of glucose
intake by peripheral tissues and the amount of glucose secretion. Gluconeogenesis
is the synthesis of glucose from non-carbohydrate precursors. It occurs
in the liver and in small amounts in the kidney, brain, skeletal muscles
and heart muscles.(1) The process of converting glucose to pyruvate is
glycolysis and this process includes three irreversible steps with very
high negative free energy in the forward reaction. In order to convert
pyruvate into glucose, the glyconeogenic pathway uses enzymes to bypass
these irreversible steps.
One of the enzymes used is Fructose 1,6-bisphosphatase (F1,6-BPase) which
is also called fructose 1,6-diphosphatase. F 1,6-BPase is found in plants
and animals and it catalyses the conversion of fructose 1,6-bisphosphate
to fructose 6-phosphate. F 1,6-BPase is a tetrameric allosteric enzyme
that uses divalent metal cofactors. Its activity is regulated by levels
of Adenosine Monophosphate (AMP), fructose 2,6-bisphosphate, and Citrate.
Deficiency of F 1,6-BPase can lead to infantile lactic acidosis, fasting
hypoglycemia, and metabolic acidosis. |
F 1,6-BPase is an allosteric enzyme classified as a hydrolase with Enzyme
Commission designation of EC
3.1.3.11 and a Protein Data Bank identification code 1umg.(2) Its ligands
are Fructose 1,6 bisphosphate (2FP), 2-methyl-2,4-Pentanediol (MPD) and
the cofactor magnesium 2+ ion (MG). (2) F 1,6-BPase works by catalyzing
the exergonic hydrolysis of phosphate ester at the first carbon (C1) of
fructose1,6-bisphosphate to form fructose 6-phosphate and orthophosphate
Pi, as shown in the figure 1.
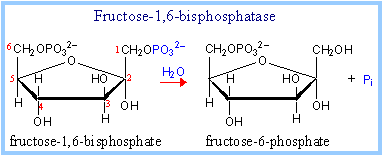 |
| Figure 1. Hydrolytic conversion
of fructose 1,6-bisphosphate to fructose 6-phosphate and Pi by fructose
1,6-bisphosphatase. © 1998-2005 (Joyce
J. Diwan). |
|
|